Why are high nitrate or nitrogen concentrations in water a problem
Why are high nitrate or nitrogen concentrations in water a problem, and what can be done to maintain safe levels?
Q:
Why are high nitrate or nitrogen concentrations in water a problem, and what can be done to maintain safe levels?
A:
Nitrate (NO3) is a common inorganic form of nitrogen. Chemically, it is an anion with a single negative charge, consisting of one atom of nitrogen and three atoms of oxygen. Because it is an anion, it is soluble in water. Plants normally use nitrate, as their source of nitrogen which is needed by all living things, and so nitrate is considered a nutrient for plants.
Nitrate molocule
Excessive concentrations of nitrate
Excessive concentrations of nitrate in lakes and streams greater than about 5 milligrams per liter (measured as nitrogen), depending on the water body, can cause excessive growth of algae and other plants, leading to accelerated eutrophication or “aging” of lakes, and occasional loss of dissolved oxygen.
Animals and humans cannot use inorganic forms of nitrogen, so nitrate is not a nutrient for us. If nitrate/nitrogen exceeds 10 milligrams per liter in drinking water, it can cause a condition called methemoglobinemia or “blue baby syndrome” in infants. Some recent studies have indicated a possible connection between elevated nitrate concentrations and cancer. Nitrate concentrations in drinking water can also have detrimental effects on the elderly with poor respiratory systems.
How do nitrates get into water?
Nitrate can get into water directly, as the result of runoff of fertilizers containing nitrate. Some nitrate enters water from the atmosphere, which carries nitrogen-containing compounds derived from automobiles and other sources. Nitrate can also be formed in water bodies through the oxidation of other, more reduced forms of nitrogen, including nitrite, ammonia, and organic nitrogen compounds such as amino acids. Ammonia and organic nitrogen can enter water through sewage effluent and runoff from land where manure has been applied or stored.
Water quality and regulation
Water quality regulatory agencies seek to avoid high concentrations of nitrate in water to minimize the problems mentioned previously.
Nitrate standards take two forms:
Drinking water standards, designed to prevent adverse human health effects.
Ambient water standards, designed to prevent excessive eutrophication in lakes and streams.
Drinking water standards for nitrate, have been around since at least 1974, when the Safe Drinking Water Act was passed, and probably well before. States may set their own drinking water standard for nitrate, but most or all use the EPA standard of 10 milligrams per liter (measured as nitrogen).

Ambient water standards have also been around for years, but each State has decided on what standards to use, if any. The EPA is just now setting guidelines for determination of ambient nitrate standards for different water bodies in different regions. General information of EPA’s programs for water-quality standards and criteria is available at:
http://www.epa.gov/waterscience/standards
Keeping drinking water free of Nitrates
Keeping drinking water free of excessive concentrations of nitrate involves a multiple barrier approach. The most effective strategy is prevention by, keeping chemicals that contain or can generate nitrate out of the water. This means managing agricultural operations to minimize application of fertilizer and to minimize runoff of fertilizer that is applied.
Nitrate control by farmers
Some farmers are now using computerized maps of their fields, calibrated to the specific soil and water conditions in various parts of their fields, to restrict the application of fertilizer to only what is needed for each part of the field. In some countries, for example Switzerland, drinking water providers enter into contracts with farmers in their source areas in which farmers receive subsidies to eliminate fertilizers and use organic farming methods.
Other nitrate control methods
Nitrate contamination prevention also means, proper handling of manure and animal waste lagoons, to minimize the discharge of animal waste or waste runoff to streams. Nitrate contributions from other sources can also be curtailed, for example, by adding tertiary treatment, or by nutrient removal, to sewage treatment plants, and by controlling emissions from automobiles.
In addition to prevention, drinking water providers may use advanced treatment techniques to remove nitrate from water. For example, advanced ion exchange technology to remove excess nitrate and remain below the 10 mg/L standard. In a typical year, this is needed mostly during the spring, following spring runoff after the application of fertilizer.
A good article about the occurrence of methemoglobinemia can be found at:
http://books.nap.edu/books/NI000114/html/29.html
Other nitrate studies
Beyond the scope of this post, there have been other studies conducted with dietary nitrate supplements and the effects on humans. However, the tests being conducted, are using dietary forms of nitrate such as, sodium or potassium nitrate or increasing dietary nitrate through the consumption of beetroot juice.
Studies in humans demonstrate a progressive rise over time in plasma nitrate and nitrite concentrations after oral administration of sodium or potassium nitrate. We investigated the possibility that a similar increase in these anions can be achieved by consuming dietary nitrate through the consumption of beetroot juice and that this will acutely lower arterial BP, supplement endothelial function (measured by flow-mediated dilatation, FMD) during ischemia, and inhibit platelet aggregation as a result of bioconversion to NO.
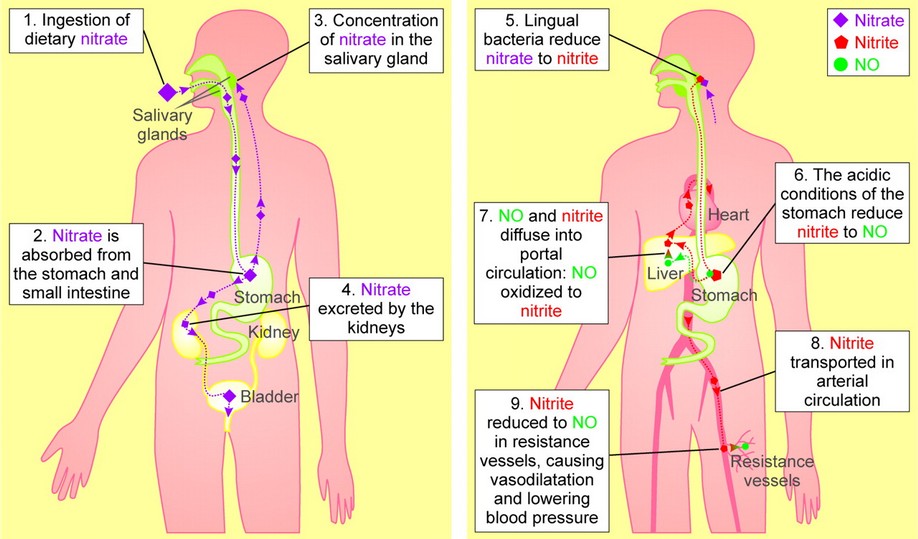
Supplemental nitrate effect study
For more information on nitrate as a dietary supplement see:
